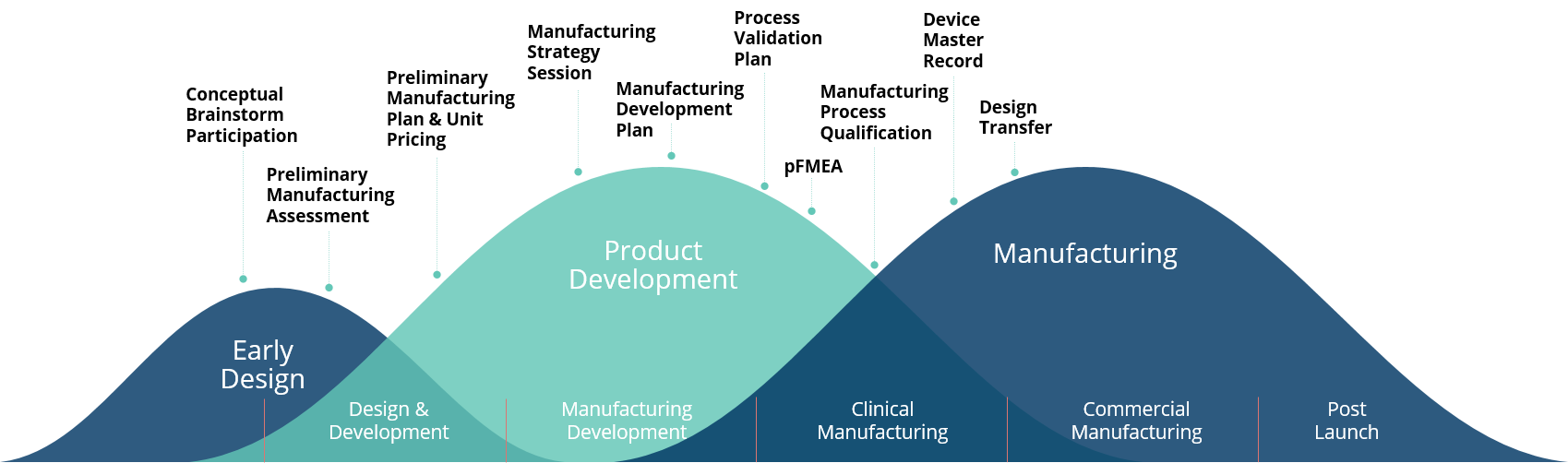
Our engineering and process expertise includes capabilities to support your early design efforts, design and development of the final design, manufacturing process development to support scale manufacturing and post launch services.
Collaboration Value